Location: Cambridge, MA
Collaborators: William Bonner, David Buckley Borden, Kennedy Rauh, and Isenberg Projects
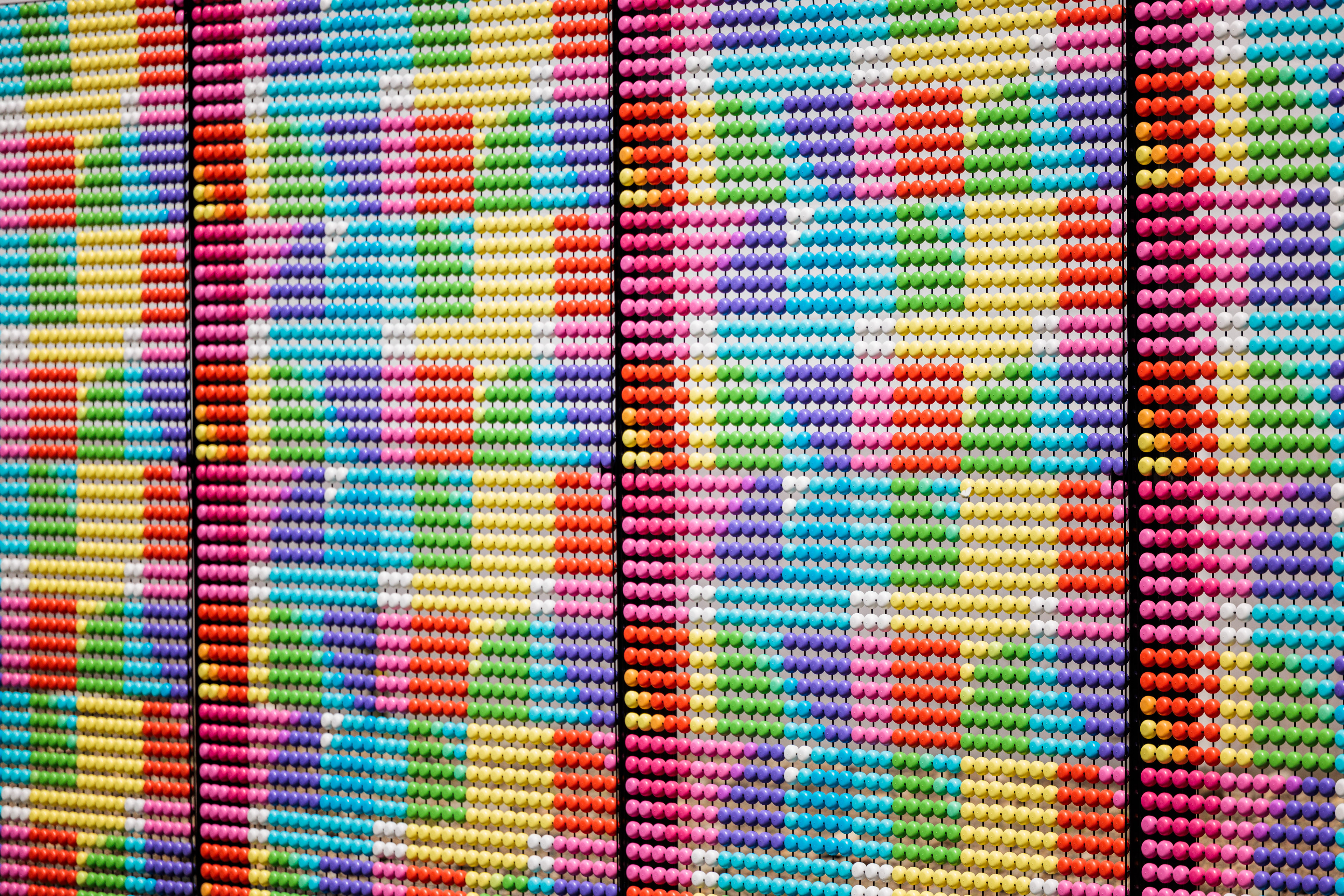
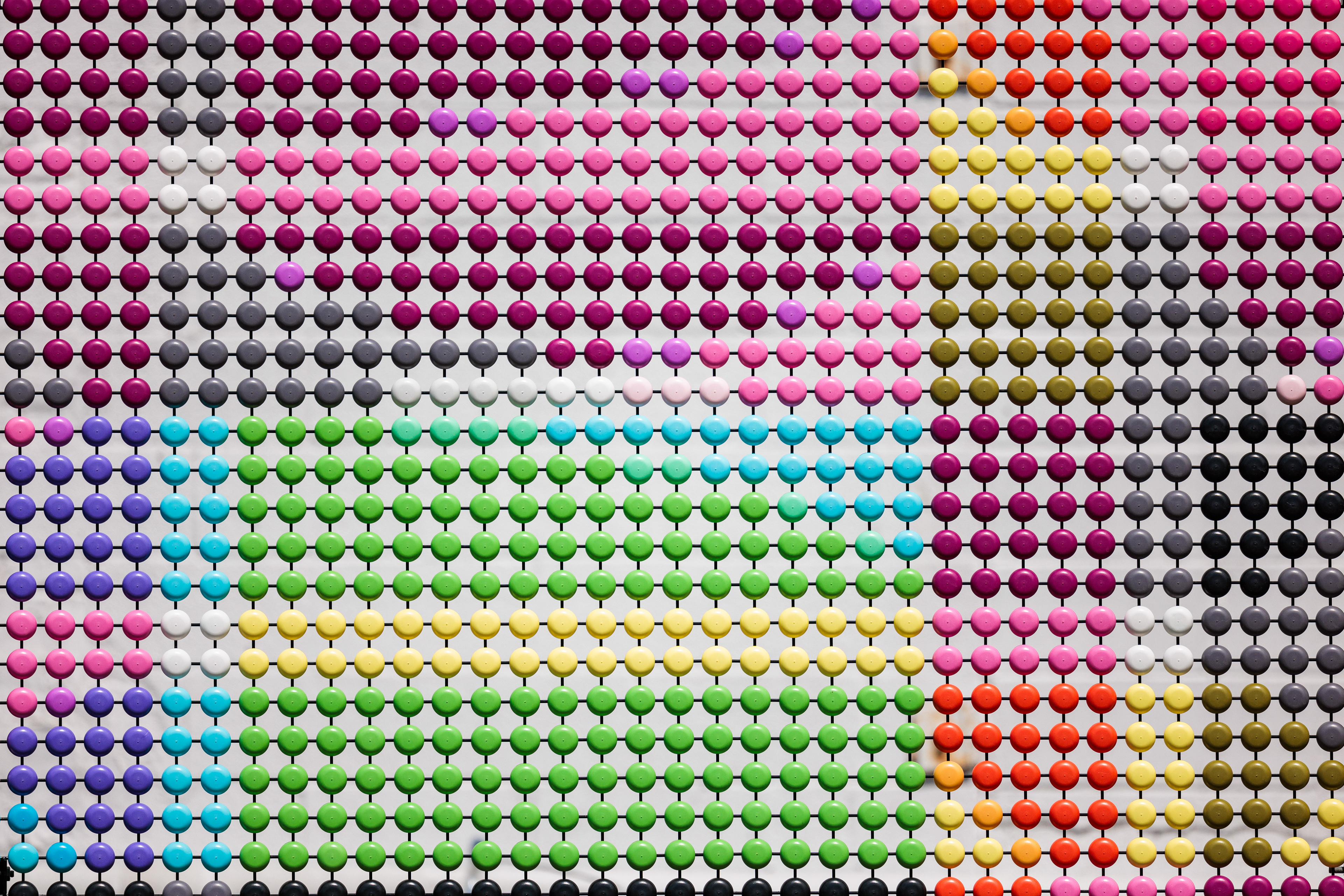
Dotted Sequences is a permanent public art project installed in the heart of Cambridge, Massachusetts, in 2024. The collaboration showcases a coded graphic pattern inspired by the fundamental building blocks of DNA as a 1500-foot long super graphic.
The primary goal of this sci-art installation is to make the complex realm of genetic disorders more approachable and engaging through public art. Dotted Sequences assigns specific pairs of colors to each rung in the spiral ladder, or double helix, that makes up human DNA. Its building blocks are adenine (A), thymine (T), cytosine (C), and guanine (G) and are represented by the colors cyan, black, magenta, and yellow, respectively. The approximately 3 billion rungs (or base pairs of nucleotides) that compose the human genome are organized across the 23 pairs of chromosomes found in nearly every cell of the human body.
The primary goal of this sci-art installation is to make the complex realm of genetic disorders more approachable and engaging through public art. Dotted Sequences assigns specific pairs of colors to each rung in the spiral ladder, or double helix, that makes up human DNA. Its building blocks are adenine (A), thymine (T), cytosine (C), and guanine (G) and are represented by the colors cyan, black, magenta, and yellow, respectively. The approximately 3 billion rungs (or base pairs of nucleotides) that compose the human genome are organized across the 23 pairs of chromosomes found in nearly every cell of the human body.
DNA, “the molecule of life,” is a chemical code that holds the instructions for how all living things grow and function. The CMYK color-coded pattern transliterates this intricate language to represent significant sections of code that have been associated with specific genetic diseases. For each disease represented, local scientists, institutions, and biotechnology companies have made significant strides in identifying and researching these unique genetic mutations and their physical manifestations. By developing a colorful, coded visual language that symbolizes actual gene sequences responsible for these diseases, the installation acts as a communication link between the scientific community and the public of Cambridge.
The color sequences should be read clockwise, starting at the 12 o’clock position. Reference diagram to right.





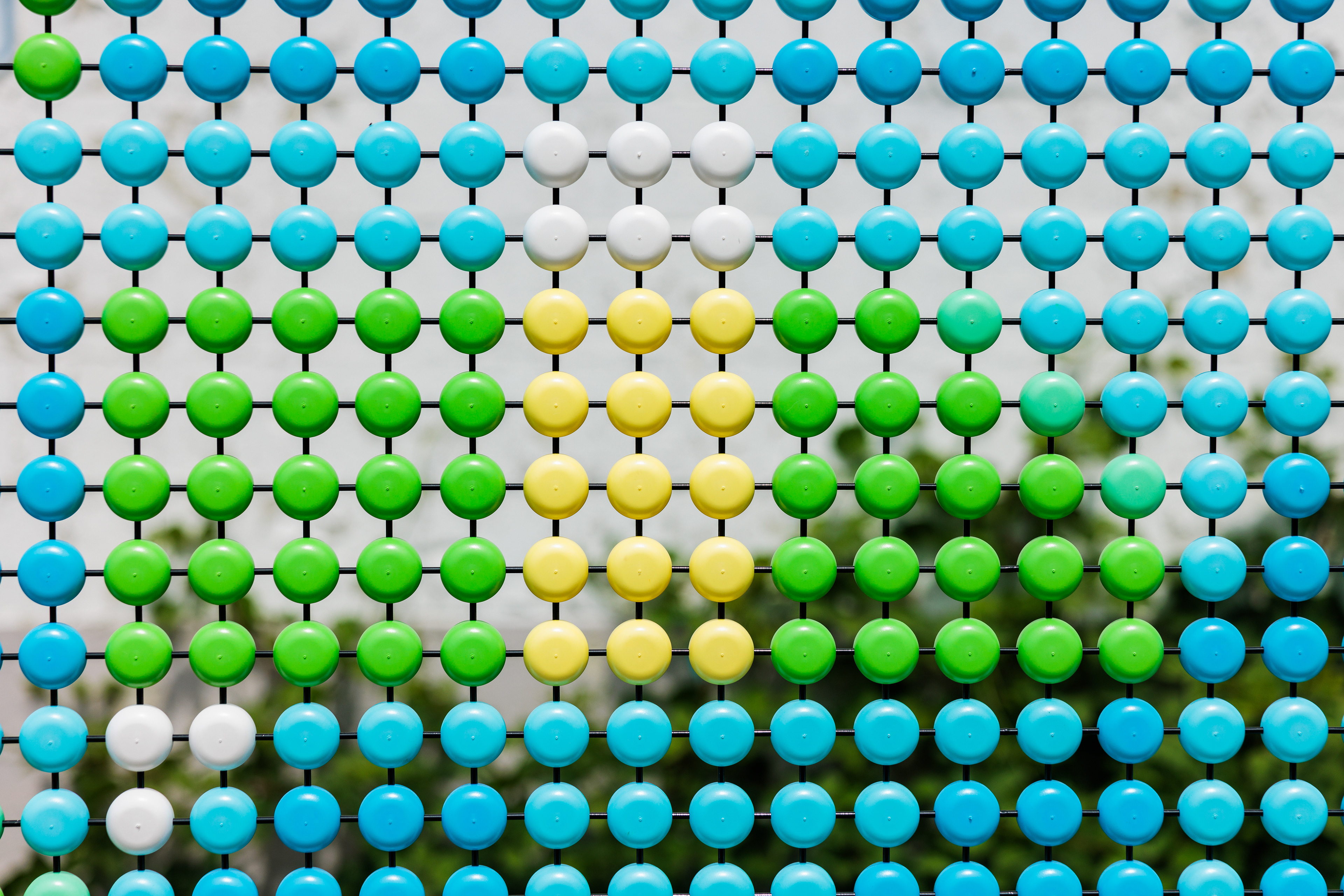

HUNTINGTON’S DISEASE (1983)
The first genetic marker for a neurodegenerative disease is mapped by James Gusella and his lab at Massachusetts General Hospital and Harvard Medical School. The initial groundbreaking discovery by local scientists, aided by the research of Nancy Wexler of Columbia University and others, links Huntington’s disease to chromosome 4 and launches a hunt for the specific gene. Ten years later, the Huntington’s Disease Collaborative Research Group will successfully isolate the HTT gene and identify that mutations at a particular site causing a trinucleotide CAG sequence to repeat beyond about 40 times are causative for Huntington’s disease.
The installation depicts the mutation, indicated by the CAG trinucleotide repeating 40 times.
GAUCHER DISEASE (1986)
A Kendall Square biotechnology company initiates development of an enzyme replacement therapy to address the deficiency of glucocerebrosidase caused by mutations in its gene GBA1. The pioneering drug, Ceradase, will receive FDA approval in five years (1991) and mark a significant milestone in the treatment of Gaucher disease. Following this success, the company’s first improved form of the enzyme, Cerezyme, will receive FDA approval in 1994. Both therapies will serve as mainstay treatments for the characteristic manifestation of Gaucher, the accumulation of lipids in the spleen, liver, and bone.
The installation consists of a vertically mirrored pattern to represent the autosomal recessive inheritance of the genetic disorder, which requires both parents to pass a copy of the mutation for the disease to manifest in their offspring.
MULTIPLE SCLEROSIS (1996)
A Kendall Square biotechnology company pioneers the development of Avonex, a drug that slows the progression of MS, an autoimmune disease where the immune system attacks the insulating sheath around nerve fibers, slowing the speed of electrical impulses in the central nervous system. Clinical trial data will show a slower disease progression over 15 years among patients who received its treatment.
The installation mimics disruptions in communication between brain and body. A genetic marker for MS is yet unknown, but manifestation of the disease is likely the result of a combination of genetic susceptibility and environmental triggers.
AMYOTROPHIC LATERAL SCLEROSIS (2012)
Multiple Kendall Square biotechnology companies are researching ALS, the most common progressive neurodegenerative disease among motor neuron diseases. In 2023, one of the treatments developed by those companies, Qalsody, became the first FDA-approved drug to target a genetic cause of ALS.
The installation represents the CCCCGG hexanucleotide repeat segments that are associated with another form of this disease. With normal repeats ranging from 2 to 24, repeats that exceed 30 are considered pathogenic and can be as high as 4,000.
AMYOTROPHIC LATERAL SCLEROSIS (2012)
A Cambridge-based company successfully treats the first patient with a gene therapy for Sickle Cell disease. The genetic disorder, which alters the shape of red blood cells, inhibits blood’s ability to transport oxygen throughout the body and can lead to painful and potentially life-threatening blockages of blood flow. Sickle Cell is the result of a single mutated nucleotide from GAG to GTG. In 2023, the first gene therapies for sickle cell received FDA approval for their curative potential for this disease.
The installation represents this CAG-to-GTG mutation by three filled-in nucleotides.
CYSTINOSIS (2019)
A Kendall Square company successfully treats the first patient with a genetic therapy for cystinosis, a disorder that leads to the buildup of the metabolite cystine within cells. Cystinosis impacts various tissues and organs in the body, most commonly the kidneys and eyes, and is caused by a mutation in chromosome 17, at the CTNS gene. This therapy is still being evaluated in clinical trials.
The installation’s horizontally mirrored pattern represents bi-allelic mutations, which require mutations in a particular gene to be passed down from both parents to occur and can cause recessive genetic diseases like cystinosis.
FRIEDREICH’S ATAXIA (2020)
The Broad Institute establishes a highly collaborative accelerator to explore potential treatments for Friedreich’s ataxia, a progressive neurodegenerative disorder that manifests in early adolescence. The mutation in the FXN gene has been known since 2000, but the mechanism of disease remains unclear and there are no approved treatments for this most common of the inherited ataxias. The mutation of trinucleotide GAA that causes it to repeat beyond its normal 5–33 times hinders the production of the FXN protein in the mitochondria, the cell’s energy-generating plant.
The installation depicts the trinucleotide GAA repeating an expanded 96 times.
THE CODON DECODER
Much like a word (and its meaning) is determined by the order of its letters, so is a gene (and the genetic information encoded within it) determined by the arrangement of its nucleotides. The arrangement of these nucleotides, which make up a gene, a chromosome, and the entire genome, is determined by scientists through a process known as DNA sequencing.
A codon is a sequence of three nucleotides that codes for, or instructs cells to synthesize, a specific amino acid in a process called translation. Through this process, the genetic code of DNA, which is made up of many codons, is translated into proteins, which are made up of many amino acids. By convention, each amino acid corresponds to a specific letter of the alphabet.
Use the decoder to the right to discover hidden phrases within portions of the fence.